The Shock of the New: Translating the Potential of Regenerative Medicine Technologies
As published in Australasian Biotechnology, Vol 26 No 3 in October 2016.
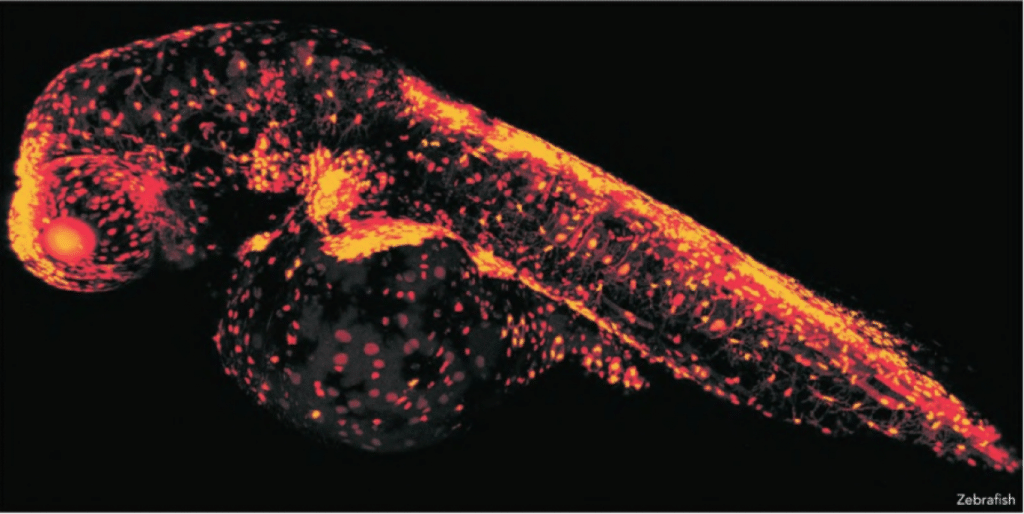
More than a decade ago, scientists around the world confidently predicted that stem cell research would revolutionise medicine. Enabling legislation followed the technology, as did a dramatic increase in funding that paralleled the public’s impatient expectations. It’s only within the past couple of years that we have finally started to see the vanguard of this regenerative movement begin to be realised – what began as a trickle of clinical trials has now become a steady stream around the globe.
Australia’s investment in regenerative research began almost 20 years ago. As the years have passed, skills have expanded, international and local networks have deepened, and fundamental knowledge has been accumulated—and a significant commitment to funding has been delivered. Australia stands ready to see the investments of the past come to term, translating into a rich stream of commercial opportunities.
The Australian Regenerative Medicine Institute (ARMI), opened in 2009, and is now a major contributor to advances in regenerative medicine research. Based at Monash University, the Institute is investigating how the body repairs, replaces, restores or regenerates damaged tissues and organs. This work could hold the key to helping unlock the body’s own potential to heal and to regenerate back to optimal health after an injury or disease. Imagine the implications!
Commercialising the regenerative research revolution starts now
ARMI is a young institute by any benchmark. And when you’re young, the focus is naturally on creativity, pace and entrepreneurship. As with any young organisation, ARMI has a strong desire to make its mark and to deliver a ‘score on the board’ as soon as possible—securing the Institute’s future and demonstrating value to stakeholders and to those patients watching with impatient interest.
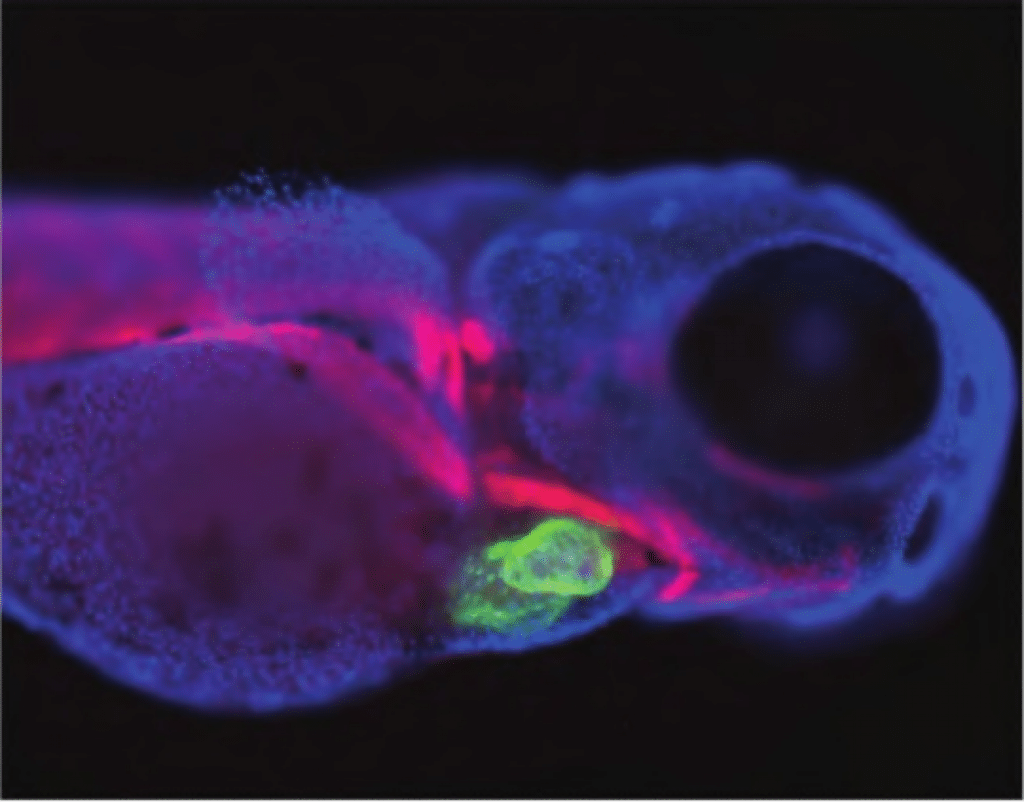
‘Translation, commercialisation and expansion are increasingly among the most common themes discussed [by] the leadership group at ARMI,’ says director, Professor Peter Currie. ‘More and more, we recognise the intense interest that scientists at ARMI have in seeing their research outcomes advance to clinical development, and we are impatient to build relationships with those in the industry sector who can shape the next stage of our development as an organisation, as well as accelerate our discoveries, towards a clinical impact.’ Many ARMI research programs are now ready to accelerate into the commercial sphere, including software packages, treatment strategies, drug discovery platforms and specific biologics.
ARMI’s goal in the coming year is to harness the wealth of experience, networks and capabilities of Australia’s biotechnology industry sector, particularly those aspects focused on regenerative medicine, to deliver on the regenerative medicine promise. The Regenerative Medicine Advisory Group, chaired by ARMI chief operating officer Silvio Tiziani, is shaping the engagement model by which industry and academia can come to the table as equal partners. Programs will be formed to provide greater visibility and access to research expertise and infrastructure, fully utilising the support programs offered by government. By coordinating and leveraging our collective commercialisation experience and skill, we will deliver the greatest benefits, faster.
Tapping into the global regenerative medicine and venture capital networks
ARMI recently forged links with a successful international player in the regenerative medicine commercialisation space that has a well-established commercialisation model, including an industry consortium and venture capital funding. The Centre for Commercialization of Regenerative Medicine (CCRM) in Canada has formed a robust framework for acceleration of regenerative medicine discoveries, and is keen to help establish a similar model in Australia. ARMI believes that the feeling is mutual.
The Australian link to the CCRM is taking shape rapidly, and plans are now underway to launch the CCRM model in Australia. When connected to other hubs also being established in other regenerative medicine powerhouses, including Israel, Japan and Singapore, this will lay the foundation of a translation superhighway—the next logical step in bringing regenerative medicine into the clinical domain.
‘With Australia’s track record of excellence in clinical trial infrastructure and skills, I see a big future for the rapid acceleration of regenerative technologies into development,’ says Tiziani. ‘I’m keen to see our clinical trial sector and biotech industry fully engaged and utilised in working with us to achieve what we have all been promising for years. The hard work is only just beginning.’
The Australian Regenerative Medicine Institute is supported by grants from the Victorian Government and the Australian Government.
